- Home
- Article
Keeping Infection Control Top of Mind
By: Carol Katarsky | Contributing Editor
Published: 2/9/2023
Accreditors are making disinfection practices a priority in a post-pandemic world — so should you.
Infection prevention procedures have always been a key focus during on-site inspections, and their importance has only intensified since the pandemic began in 2020. Here’s what surveyors will be looking for — and the steps surgical centers can take now to ensure they pass those inspections with flying colors.
The COVID effect
Be prepared to get peppered with questions about your facility’s COVID-19 policies: “What did you do during the pandemic? How did you protect your patients? How did you protect yourself? What did you do with visitors during that time?” says Kathy Beydler, RN, MBA, CNOR, CASC, principal consultant at Whitman Partners, a search firm that places perioperative directors at ASCs and HOPDs. Ms. Beydler notes that having a pandemic-ready infection prevention policy in place isn’t enough: Surveyors want to see how you dynamically pivoted and changed it since 2020 as more was learned about COVID that triggered new recommendations from health officials and regulators.
Key areas of concern
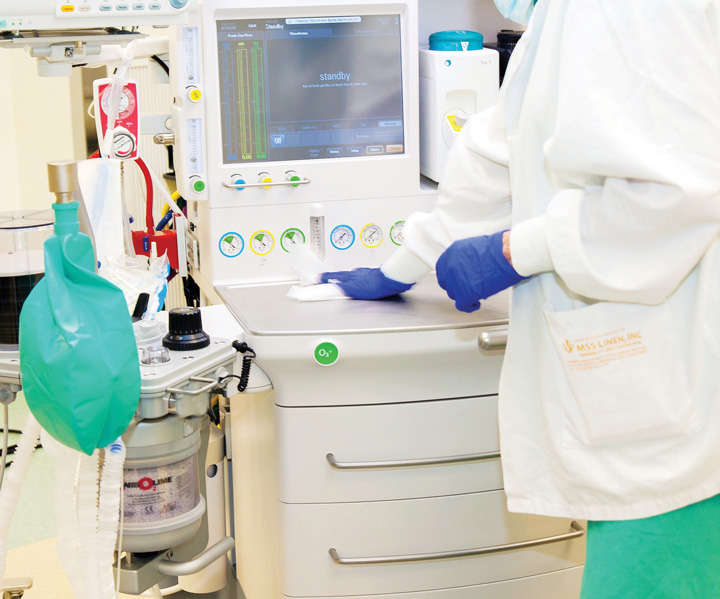
Some of the crucial components surveyors will focus on include injection and medication practices, hand hygiene, how well your facility is cleaned and maintained, sterile processing functions and staff training levels on infection control practices. The most basic tasks are often the ones that facilities don’t do well.
Staff can become complacent over time about the risks of not paying attention to details of infection prevention measures by pressure, be it real or perceived, to maintain or reduce turnover times. Employees sometimes cut corners, go on autopilot, forget their training or revert to a prior way of doing things. “Sometimes, they’ve been doing it for so long and it’s a habit they just don’t think about,” says Ann Geier, MS, RN, CNOR(E), CASC, chief nursing officer at Ambulatory Healthcare Strategies, a regulatory and financial oversight organization. Whatever the cause, missteps surrounding the little things can lead to getting dinged during an inspection because patient safety has been put at risk.
Injection and medication practices are one of the first areas a surveyor will examine — and violations in this area can result in immediate citations and shutdowns. Common issues surveyors check for include reuse of needles, disinfection of septums on medication vials, how spiked IV bags are handled, as well as proper procedures for single- and multiple-use medication vials.
“For example, let’s say I’m doing a survey and I see your nurse anesthetist use a syringe to draw a med, put it in her drawer and not label it. If an hour later she uses that syringe, that’s considered immediate jeopardy,” says Ms. Geier. Ms. Beydler says she sees a lot of errors made with multi-use medication vials in the OR or at the bedside. “CMS has said only single-use vials can be used there, she says. “If you’re using a multi-use vial, it has to be accessed outside the patient care area.”
Ms. Geier notes many of the injection and medication mistakes are made by anesthesia providers. “The protocols have changed over the years and things we used to do are not allowed now. I know that anesthesia feels put upon but that’s the way it is,” she adds.
Hand hygiene is another area that is fairly easy to get right but can still trip up even veteran staff. “We’re just looking for compliance with hand hygiene protocols, such as glove-wearing and handwashing,” says Ms. Geier.
An ‘environment of care’
The basics of keeping facilities clean also tend to trip up many outpatient centers. “Have you been terminally cleaning your operating rooms? If I run my finger over the surface in the operating room, am I going to find dust?” explains Ms.
Beydler. “For surveyors, a clean facility goes a long way toward letting them know how you take care of your patients.”
Ms. Geier emphasizes the importance of cleaning the entire facility. “We look under stretchers and behind things, just to see if somebody moved the item so that they could thoroughly clean. We’re looking for crud that’s accumulated on the floors or in the corners,” she says. “I know no facility has enough storage, but if they have a lot of clutter then they’re probably cleaning around the clutter rather than cleaning everywhere.”
Surveyors are also looking at whether cleaning protocols are being followed, and to what degree. For example, staff can’t go through the motions and not wait for the specific amount of contact time required by the cleaning solution they’re using.
Even issues that may seem cosmetic, such as chipped paint, chipped or stained ceiling tiles, or dings and cracks in the wall, can harbor dirt and bacteria and be flagged in a survey.
Ms. Beydler says a common issue she’s seen lately is missing drip pans under hand sanitizer dispensers. The resulting residue can create a place for dirt to accumulate or, if it drips on the floor, could be a fall hazard. Other potential red flags that are harder to see include dirty air vents, obstructed sprinkler heads, fire extinguishers that haven’t been properly serviced, as well as blocked access to electrical panels or gas shutoff valves. Even though your staff may not be able to identify and fix all of these issues themselves, it’s still a good idea to huddle with facility management and housekeeping before a survey to examine these areas before the inspectors do.
Prepare well in advance

With so many details to nail down and training to reaffirm, facilities should start preparing for their survey well in advance of when it’s expected. “You think you’ve got all the time in the world until you start finding things that are wrong,” says Ms. Geier. “By then there might be no time to fix them.”
Depending on the type of survey and the size of the center, the time to start your preparation is six to 12 months before a survey. Some centers may consider doing it yearly to ensure their procedures stay tight. “Some places will wait until the last three months before Joint Commission is due, which is too late,” notes Ms. Beydler.
Ms. Geier and Ms. Beydler recommend doing some form of a mock survey — whether it’s an internal version, working through the CMS infection prevention worksheet or having someone from outside come in to review potential problem areas and suggest ways to improve them. In either case, it’s important to involve all staff in the process to best identify problems and produce solutions. It also helps to think of a survey as more of a professional collaboration than a test you might fail.
Surveyors want to help centers operate more smoothly and safely. If they’ve identified an area where you’re struggling, they’re prepared to help you with it.
Ann Geier, MS, RN, CNOR(E), CASC
“Staff sometimes thinks it’s going to be a ‘gotcha’ game, but it’s really not,” says Ms. Geier. “Surveyors want to help centers operate more smoothly and safely. If they’ve identified an area where you’re struggling, they’re prepared to help you with it.”
Keep all levels of disinfection in mind
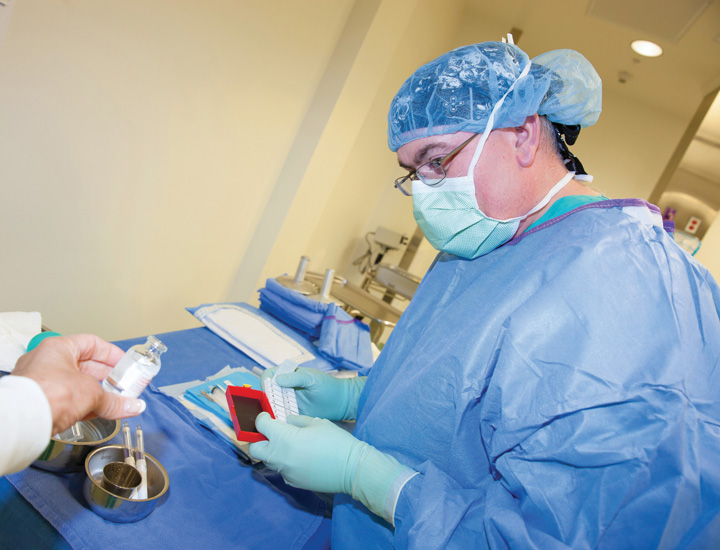
Make sure to bone up on best practices regarding low-, intermediate- and high-level disinfection, as well as sterilization protocols before your survey.
Low-level disinfectants don’t kill microbacteria, while intermediate-level disinfectants do. They are often used interchangeably by healthcare facilities, with many opting for products that include intermediate-strength disinfectants. They mostly come in the form of premoistened wipes, or concentrated sprays or foams, and are used for routine environmental cleaning of noncritical equipment, such as horizontal surfaces, blood pressure cups, IV poles — any multipatient-use item that doesn’t come into contact with broken skin or mucous membrane.
High-level disinfectants make kill claims to eliminate everything except large numbers of bacterial spores and are used on reusable semi-critical equipment such as endoscopes and endocavity ultrasound probes. Sterilization kills everything and is used for surgical instruments, implants and equipment used in eye procedures.
Many deficiencies are often found in sterile processing departments. “We look very closely to make sure that correct SPD processes are in place,” says Ms. Beydler. “Many times, the infection preventionist is not an OR nurse so the SPD can kind of fly under the radar, and everything doesn’t get done according to manufacturers’ instructions for use.”
Finally, surgery center teams must always remember that patients are put at risk when infection control practices fall short. “We can’t lose track of the fact that these are actual people who are lying on that operating room table,” notes Ms. Beydler. “Their lives and those of the people who care about them are going to be impacted by what we do.” OSM
.svg?sfvrsn=be606e78_3)
.svg?sfvrsn=56b2f850_5)