The updated ST91 recommends that storage cabinets should be placed in a secured location, not in procedure rooms or within three feet of a sink. Visually inspect scopes and storage cabinets for cleanliness when placing the instruments in cabinets
and removing them for patient use, says Ms. Klacik, who says cabinets should be cleaned according to the manufacturer’s IFU weekly or when visibly soiled.
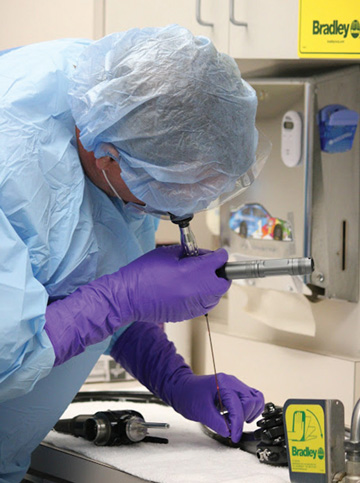
Importantly, notes Ms. Klacik, an endoscope that appears clean can harbor debris that cannot be seen without magnification. “Cleaning verification used after endoscope cleaning and before disinfection may detect residual organic soil and
microbial contamination present on a surface, even if the devices appear clean,” she says.
• No manual disinfection. The new guidance recommends against the manual high-level disinfection of scopes, in part because of the “variability and consistency in personnel responsible for the process,” according
to the new standard. “There are simply too many inconsistencies with the manual process,” says Mr. Grisby. “The manual process is only as good as the processor that is doing the job. If an employee is fatigued or not feeling
their best, the manual process might not be performed the correct way and steps might be missed. While most facilities have made the move to the automated process, globally we are not there yet. Access to the right market and limited financial
resources can get in the way.”
• Storage time. Determining the length of storage, or “hang time,” that a scope can withstand before needing to be reprocessed is a challenge for many facilities. “This is precisely the reason
why the move was made to performing a risk assessment when determining the length of hang time for scopes,” says Mr. Grisby. “There are several factors to consider.”
For example, he points out some storage locations are in high-traffic areas and others have restricted access to the cabinet. “This is why we’ve emphasized that a multidisciplinary team of stakeholders, including infection prevention,
at individual facilities should determine what, if any, risks there are in extending or shortening hang time,” says Mr. Grisby.
The updated document is intended to give facility leaders and managers a source to reference when asking frontline staff to add steps to their reprocessing routines in the interest of increased patient safety. “When they want to introduce
change in their facility that can improve safety, they might get pushback from leadership because there are few guidelines out there that say what they should do or how to do it,” says Mr. Grisby. “That’s why they need this
document to back up and outline what needs to be done.”
Balancing the scheduling of cases while allowing enough time in between procedures for proper endoscope reprocessing can be a challenge of high-volume facilities. Mr. Grisby also points to an overall lack of staff education as a potential problem
area. “The challenge is employee knowledge,” he says. “Facilities should have a training plan in place for any new hires that will handle endoscopes in the procedure room or in the reprocessing room.”
He’s found it helpful to discuss proper endoscope care during daily huddles or department meetings to ensure physicians, infection preventionists and frontline staff are on the same page. Mr. Grisby reaches out before staff or committee
meetings to make sure endoscope care is included on the agenda so he’s not mentioning the topic in the middle of a workday and interfering with patient care of the flow of busy staff members and physicians.
The new standard comes with extensive appendices citing peer-reviewed research and data to support the requirements and recommendations. “We’re not just throwing out statements just because we want facilities to do these things,”
says Mr. Grisby. “We want facility leaders and frontline staff to have the research and patient safety data at hand to advocate for change in their departments.” OSM
.svg?sfvrsn=be606e78_3)
.svg?sfvrsn=56b2f850_5)