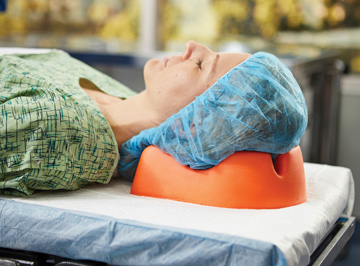
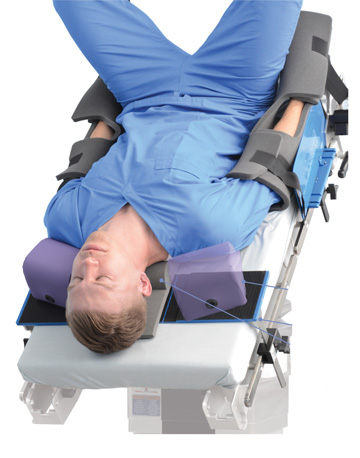
Patient positioning aids range from simple gel pads to elaborate table attachments. However, there are certain common denominators you should consider when evaluating these vital products that prevent patient injuries and give surgeons the access they need to operate safely and effectively.
- Due diligence. Contact vendors and request to speak with personnel at facilities that currently use the devices you’re evaluating to ask about their real-world experiences with them. Also, examine the evidence on the devices’ safety, efficacy and incidences of injury. Would your OR team need to stand in awkward, stressed positions at the surgical field because of the device’s design? If something rigid on the device sticks out, could it lead to injuries among patients or staff?
- Materials. Determine how the device will hold its shape and density, and if it generates heat or promotes moisture development, while keeping in mind that all of these are multifactorial issues. For example, a device made of heavy vinyl-type plastic tends to build up more heat, which may lead to patients perspiring. Moisture on patients’ skin can lead to breakdown and pressure injuries. Overarching issues with mircroclimate can be exacerbated when warming devices are used, because heat can build up between the warming device and the positioning device. Consider patients’ known and possible allergies to certain device materials.
- Single-use versus reusable. Beyond financial considerations, the debate about single-use versus reusable devices touches on storage space and environmental stewardship. For example, will your staff have the time and resources to handle reusable devices carefully enough to promote longevity? Consider that reusable devices often actually have a limited amount of uses, which vendors and operational manuals should specify.
- Storage. Outpatient surgical facilities are commonly challenged in terms of space, which makes where and how you’ll store positioning devices a primary consideration. Take a close look at your storage space. For example, capping sharp edges on shelves can prevent damage to positioning devices. Also consider investing in rolling carts to transport these devices in order to relieve physical burdens on your team and to maintain device integrity.
- Follow directions. A perfectly good positioning device can turn into a dangerous one if it’s not used properly. Following the manufacturer’s instructions for use and procedure guide, as well as your facility’s
policies and procedures, should protect the patient from harm. Conduct education and periodic reeducation to promote following the manufacturers’ instructions for use to the letter in terms of how these devices are cleaned, used
and set up.
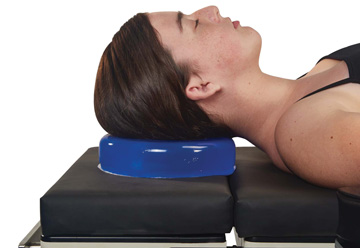
Misuse of patient positioning devices is widespread. Take gel pads, for example — some providers regularly place layers of materials between the gel and the patient, even though the instructions typically say to place the patient directly on the gel. While providers believe they are doing a good thing, they are not, often due to lack of education. Trial and consider a device’s limitations. For example, will it impede the surgeon from visualizing anatomy on an intraoperative X-ray, or make it more difficult for staff to find a retained object? - Involve everyone. Nurses and surgical techs are often responsible for gathering, assembling, using and cleaning the devices before and after cases. Surgeons should also be involved in device selection and educated on the use of devices before purchasing decisions are finalized. All OR personnel who use, transport and maintain positioning devices, as well as the financial stakeholders, should be involved when products are trialed and selected. OSM
.svg?sfvrsn=be606e78_3)





.svg?sfvrsn=56b2f850_5)