Proper control of S. aureus colonization prior to surgery is an important, sometimes overlooked step in reducing the SSI risk associated with S. aureus carriage in the nares.
 Credit: 3M Company
Credit: 3M Company
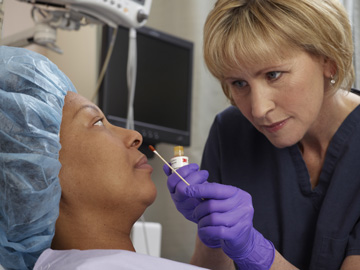
Application of 3M™ Skin and Nasal Antiseptic (Povidone-Iodine Solution 5% w/w [0.5% available iodine] USP) Patient Preoperative Skin Preparation to patient.
 Credit: 3M Company
Credit: 3M Company
Kimberly Prinsen, RN MSN.
In the US alone, combined inpatient (10,303,000) and outpatient (11,474,800) surgeries account for approximately 22 million surgical procedures per year.1 Each procedure can place a patient at risk for a surgical site infection (SSI). It is well documented that S. aureus is the most common cause of SSIs and is responsible for 30.4% of infections.1 A substantial proportion of these (43.7%) are caused by methicillin-resistant S. aureus (MRSA).2 Overall, S. aureus SSIs result in considerable morbidity and mortality,3 yet research indicates that up to 60% of SSIs are preventable.4
The importance of nasal decolonization to combat SSIs cannot be overstated and guidelines are evolving to include nasal decolonization as part of a comprehensive preoperative protocol to help reduce patients’ risk of SSI. In the 2019 update of the CDC Strategies to Prevent Hospital-onset Staphylococcus aureus Bloodstream Infections in Acute Care Facilities, surgical site infection (SSI) prevention practices were addressed. The guidance recommended as a core strategy that all patients undergoing high risk surgeries (e.g., cardiothoracic, orthopedic and neurosurgery), unless known to be S. aureus negative, use an intranasal antistaphylococcal antibiotic/antiseptic (e.g., mupirocin or iodophor) and chlorhexidine wash or wipes prior to surgery.
Facilities can choose to apply the selected pre-operative source control regimen universally to all patients versus screening for MRSA and treating. Recently, in alignment with the CDC guidance, universal nasal decolonization was included in the AORN Guidelines for Perioperative Practice: Patient Skin Antisepsis (May 2021). This focused attention on the need to decrease bacterial load, particularly S. aureus, especially in high-risk surgical procedures as more complex procedures, such as ortho and spine, move to the ambulatory setting. The guideline also focused on PVP-I as an alternative to an antibiotic (mupirocin).5
Keep in mind that high risk patients are not limited to cardiothoracic, orthopedic and neurosurgery, nor are they limited to inpatient surgery. In addition, there continues to be movement of more complex procedures being done in the ambulatory setting. For example, procedures that include implants increases risk for SSI and can occur in many specialties. Per the AORN guidance the need to include the decolonization as part of a preoperative protocols should be evaluated by an interdisciplinary team.5
Reducing SSI risk
The human body is a reservoir for S. aureus. Approximately 30% of healthy adults are carriers.3, 6, 7 In fact, S. aureus carriers are two to nine times as likely as non-carriers to develop SSIs, suggesting that the majority of infections are caused by a patient’s endogenous bacteria, including S. aureus.8,9 The effective control of S. aureus colonization prior to surgery is an important, sometimes overlooked step in reducing the SSI risk associated with S. aureus carriage in the nares.
The anterior nare is recognized as an anatomical niche for S. aureus10 with its own unique bacterial ecosystem.11 Currently, the standard choice for nasal S. aureus decolonization prior to surgery is intra-nasal mupirocin (an antibiotic), applied twice-daily for 5 days prior to surgery.12 A number of studies have shown intra-nasally applied mupirocin to reduce the proportion of procedures resulting in SSIs,13,14 with one study reporting a reduction of 94% in patients undergoing gastrointestinal surgery.15 However, other randomized controlled trials have failed to show a significant reduction in infection rates.16-18
Nasal S. aureus decolonization with mupirocin has drawbacks, including patient compliance due to the required twice-daily regimen over a five-day period as well as affordability. Another concern is increasing antibiotic resistance.19 These limitations have led to the exploration of alternatives for nasal decolonization.
Finding alternatives
3M™ Skin and Nasal Antiseptic (Povidone-Iodine Solution 5% w/w [0.5% available iodine] USP) Patient Preoperative Skin Preparation, is cleared for preparation of the skin prior to surgery, as it helps reduce bacteria that potentially can cause skin infections. Safe for use in children as young as two months old (see 3M Drug Facts label), it has been available since 2010. The patented formula is specially designed for the unique nasal environment as a topical antiseptic approach to help reduce the risk factor of S. aureus that can lead to SSIs, providing an alternative to nasal decolonization with mupirocin.
This solution has been specifically formulated to reduce microbial load in the unique environment of the nares, and the efficacy of this particular preparation has been widely studied.20-24 The regimen involves a one-time application of 5% povidone-iodine solution to the anterior nares prior to a surgical procedure – and it reduces nasal S. aureus colonization for at least 12 hours.11
Additionally, 3M™ Skin and Nasal Antiseptic has been extensively researched and has more published peer-reviewed and investigator-initiated studies than any other nasal decolonization antiseptic product (as of July 2021) with more than 10 clinical studies. One study, in particular, focused on cost-effectiveness; A retrospective study conducted by Torres et al. (2016), in total knee and hip arthroplasty investigated the incidence of SSIs and cost-effectiveness of universal application of 3M™ Skin and Nasal Antiseptic versus MRSA screening followed by treatment of carriers with mupirocin. The results showed 3M™ Skin and Nasal Antiseptic was an effective alternative to mupirocin, resulting in significant cost savings and eliminated the risk of mupirocin resistance.
The ease of incorporating effective nasal decolonization into the preoperative protocol for every patient every time is an important consideration when selecting which method and product to use. 3M™ Skin & Nasal Antiseptic is a one-time application done the hour* prior to surgery resulting in a 99.5% reduction in S. aureus.25 Study results have demonstrated SSI risk reduction with the aid of this simple one-time preop application as part of a comprehensive preoperative SSI reduction bundle.21,23,26
Reducing S. aureus in the nares can help reduce the risk of SSI when done as part of a comprehensive preoperative protocol. 3MTM Skin and Nasal Antiseptic is a simple solution to help address nasal colonization of S. aureus, and the clinical evidence is continuing to mount for this safe, time-saving and effective approach which can aid in the efficiency of preoperative patient preparation.
Note: To request a sample please click here.
*The clinical significance or in vitro data is unknown.
Note: Author Kimberly Prinsen, RN MSN, has over 36 years of experience as a nurse in the perioperative specialty. Kim holds a Bachelor of Science in Nursing from Allen College in Waterloo, IA and a Master of Science in Nursing Leadership from the same institution. Kim joined 3M in 2014. She is an Advanced Clinical Application Specialist with 3M Medical Solutions Division. Her current role focusses on antiseptics including nasal decolonization and perioperative skin preparation. Her work involves both product and program development. She has presented on HAI related topics throughout the United States and, in turn, created educational content for her colleagues to present globally. Prior to joining to 3M, Kim served as a Clinical Director of Surgical Services at a Hospital in Iowa and was responsible for overall operational and financial performance for Surgery, PACU, Ambulatory Unit, Sterile Processing, Ambulatory Surgery Center and Center for Pain Medicine. Kim also served as a Lieutenant in the Navy Reserves as part of the Fleet Hospital. She is a member of AORN and APIC.
 CLEANSING BLAST Povidone-iodine nasal irrigation and sprays are being evaluated for their efficacy at lowering or removing loads of SARS-CoV-2.
CLEANSING BLAST Povidone-iodine nasal irrigation and sprays are being evaluated for their efficacy at lowering or removing loads of SARS-CoV-2.
.svg?sfvrsn=be606e78_3)



.svg?sfvrsn=56b2f850_5)