The antiseptic can quickly inactivate coronaviruses, including SARS, MERS and, yes, SARS-CoV-2.
 CREDIT: Cancer Research UK, CC BY-SA 4.0 via Wikimedia Commons
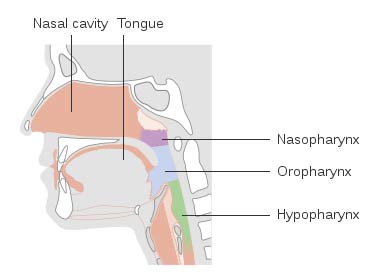
CREDIT: Cancer Research UK, CC BY-SA 4.0 via Wikimedia CommonsCOVID COLLECTOR The nasal cavities, nasopharynx, oral cavity and oropharynx can store high loads of SARS-CoV-2.
A recent literature review examined the effectiveness of nasal and oral use of povidone-iodine to reduce nosocomial spread of SARS-CoV-2, the virus that causes COVID-19. The authors concluded that using the proper concentrations of povidone-iodine in both the sinonasal and oral cavities can safely and effectively help prevent the spread of coronaviruses in general, and specifically the spread of SARS-CoV-2.
The study, published in the Ear, Nose & Throat Journal, notes that the nasal cavities, nasopharynx, oral cavity and oropharynx can house high viral loads of SARS-CoV-2, which has led to growing interest in decontaminating these areas in patients and healthcare workers to prevent the spread of COVID-19.
That led the authors to examine povidone-iodine for three reasons: its ability to inactivate coronaviruses, its lack of microbial resistance and its long history of clinical use. They reviewed its use in the nasal and oral cavities to test for its safety. Even though in surgical environments its use is episodic, the authors decided to put povidone-iodine through a more rigorous case study to determine if it was safe to use chronically during the pandemic.
The authors say povidone-iodine is effective for both preoperative and chronic nasal decolonization, with no effects on thyroid function, mucociliary clearance or olfaction. "Ciliotoxic effects have been demonstrated at concentrations of 2.5% and above, with safety demonstrated at 1.25% and lower," they add. Povidone-iodine is also used in the oral cavity for surgical disinfection, as well as chronically for dental purposes. Again, the authors say chronic oral use is safe as well, with no evident toxicity, irritation, staining of teeth or change in taste.
They do note concern regarding aspiration of oral povidone-iodine in unconscious patients, which had led to six case reports of aspiration pneumonia in surgical settings. "It is prudent to take care when instilling povidone-iodine in unconscious patients," they say.
All told, the authors say povidone-iodine is safe for nasal use up to 1.25% for five months, and safe for oral use up to 5% for six months. Based on available data, they recommend nasal mucosal decontamination with 0.5 to 2 mL of 1.25% povidone-iodine and oral rinse with up to 10 mL at 2.5% as frequently as needed for decontamination without risk of adverse effects. However, they advise against its use in patients with thyroid disease, pregnant patients and patients receiving radioactive iodine therapy.
For surgical facilities, these findings are instructive, as not only do they reiterate the general safety and effectiveness of povidone-iodine on most patients, but they also indicate that resistance is not an issue due to its effectiveness over long time periods.
.svg?sfvrsn=be606e78_3)


.svg?sfvrsn=56b2f850_5)