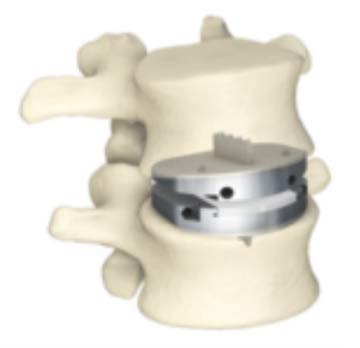
Anterior Lumbar Total Disc Replacement
The prodisc L Lumbar Total Disc Replacement system, which is the only FDA-approved implant for two-level disc replacements, is designed to restore normal disc height and treat degenerative disease from L1 to L3. Its three components and ball-and-socket design provide range of motion and stability in the replaced area of the spine, factors that reduce reoperations at adjacent levels. The implant's pronged design and plasma-sprayed titanium surface improve contact and integration with surrounding bone. It comes in 12 combinations of footprints, heights and angles for accurate matching to a patient's specific anatomy.
The Dawn of a New Era In Digital Surgery
TransEnterix has announced the FDA clearance of the Intelligent Surgical Unit (ISU) for the Senhance Surgical System, the company's first step in bringing augmented reality and machine vision learning to robotic surgery. The Senhance system currently features eye-tracking camera control; machine learning would allow the platform to recognize objects and locations within the surgical field, and guide the camera independently of the surgeon's actions. TransEnterix says the technology is forward-compatible with advanced imaging technology, including augmented intelligence, scene cognition and surgical image analysis.

Up Next in Microinvasive Glaucoma Surgery
The OMNI Surgical System from Sight Sciences lets surgeons treat points of aqueous humor outflow resistance at the trabecular meshwork, Schlemm's canal and distal collector channels. The device's next generation design includes improved ergonomics and user-friendly features based on surgeon feedback, including an enhanced method for priming the device, a flexi-grip surface on a newly designed ovoid handle for improved ergonomics, and enhanced deployment and retraction of the microcatheter to reduce intraocular pressure. The overall result is a device that's easier and more comfortable to use.
.svg?sfvrsn=be606e78_3)
.svg?sfvrsn=56b2f850_5)